This is a read-only mirror of pymolwiki.org
Difference between revisions of "Cealign"
Jump to navigation
Jump to search
Lucajovine (talk | contribs) |
m (2 revisions) |
||
| (102 intermediate revisions by 12 users not shown) | |||
| Line 1: | Line 1: | ||
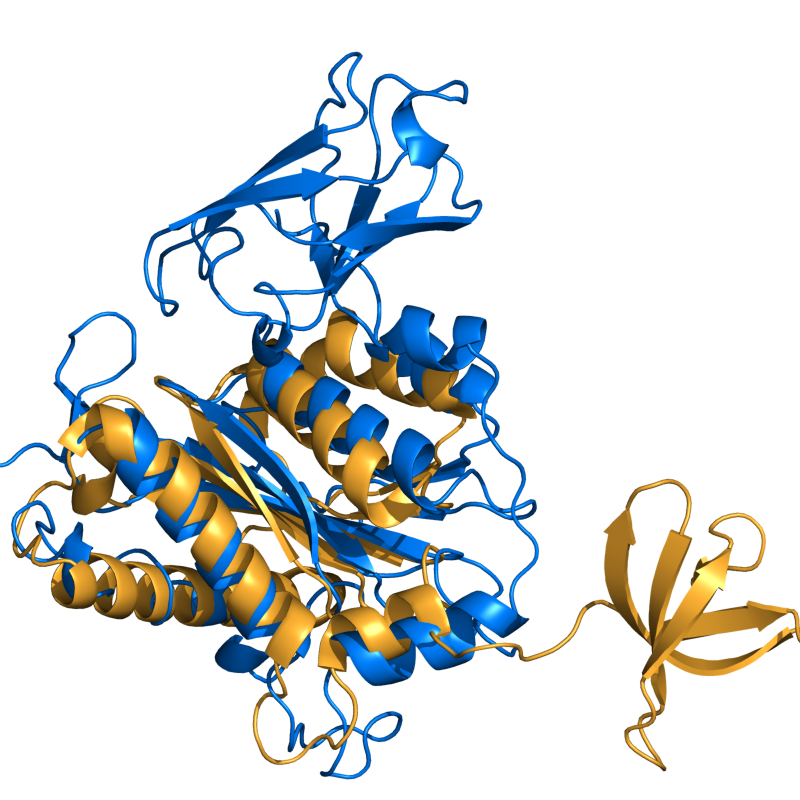
| − | + | [[Image:cealign_ex1.png|300px|thumb|right|cealign superposition of 1c0mB and 1bco]] | |
| − | |||
| − | + | [[cealign]] aligns two proteins using the CE algorithm. It is very robust for proteins with little to no sequence similarity (twilight zone). For proteins with decent structural similarity, the [[super]] command is preferred and with decent sequence similarity, the [[align]] command is preferred, because these commands are much faster than [[cealign]]. | |
| − | This | + | ''This command is new in PyMOL 1.3, see the [[cealign plugin]] for manual installation.'' |
| − | == | + | == Usage == |
| − | |||
| − | + | cealign target, mobile [, target_state [, mobile_state | |
| + | [, quiet [, guide [, d0 [, d1 [, window [, gap_max | ||
| + | [, transform [, object ]]]]]]]]]] | ||
| − | + | == Arguments == | |
| − | |||
| − | |||
| − | |||
| + | '''Note''': The '''mobile''' and '''target''' arguments are swapped with respect to the [[align]] and [[super]] commands. | ||
| − | == | + | * '''target''' = string: atom selection of target object |
| − | === | + | * '''mobile''' = string: atom selection of mobile object |
| − | = | + | * '''target_state''' = int: object state of target selection {default: 1} |
| + | * '''mobile_state''' = int: object state of mobile selection {default: 1} | ||
| + | * '''quiet''' = 0/1: suppress output {default: 0 in command mode, 1 in API} | ||
| + | * '''guide''' = 0/1: only use "guide" atoms (CA, C4') {default: 1} | ||
| + | * '''d0, d1, window, gap_max''': CE algorithm parameters | ||
| + | * '''transform''' = 0/1: do superposition {default: 1} | ||
| + | * '''object''' = string: name of alignment object to create {default: (no alignment object)} | ||
| − | + | == Example == | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | <syntaxhighlight lang="python"> | |
| − | < | + | fetch 1c0mB 1bco, async=0 |
| − | + | as ribbon | |
| − | cealign | + | cealign 1bco, 1c0mB, object=aln |
| − | + | </syntaxhighlight> | |
| − | </ | ||
| − | ==== | + | == See Also == |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * [[super]] | |
| − | + | * [[align]] | |
| − | + | * [[cealign plugin]] | |
| − | |||
| − | + | [[Category:Commands|Align]] | |
| − | + | [[Category:Structure_Alignment|Align]] | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | [[ | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 15:32, 20 October 2014
cealign aligns two proteins using the CE algorithm. It is very robust for proteins with little to no sequence similarity (twilight zone). For proteins with decent structural similarity, the super command is preferred and with decent sequence similarity, the align command is preferred, because these commands are much faster than cealign.
This command is new in PyMOL 1.3, see the cealign plugin for manual installation.
Usage
cealign target, mobile [, target_state [, mobile_state
[, quiet [, guide [, d0 [, d1 [, window [, gap_max
[, transform [, object ]]]]]]]]]]
Arguments
Note: The mobile and target arguments are swapped with respect to the align and super commands.
- target = string: atom selection of target object
- mobile = string: atom selection of mobile object
- target_state = int: object state of target selection {default: 1}
- mobile_state = int: object state of mobile selection {default: 1}
- quiet = 0/1: suppress output {default: 0 in command mode, 1 in API}
- guide = 0/1: only use "guide" atoms (CA, C4') {default: 1}
- d0, d1, window, gap_max: CE algorithm parameters
- transform = 0/1: do superposition {default: 1}
- object = string: name of alignment object to create {default: (no alignment object)}
Example
fetch 1c0mB 1bco, async=0
as ribbon
cealign 1bco, 1c0mB, object=aln